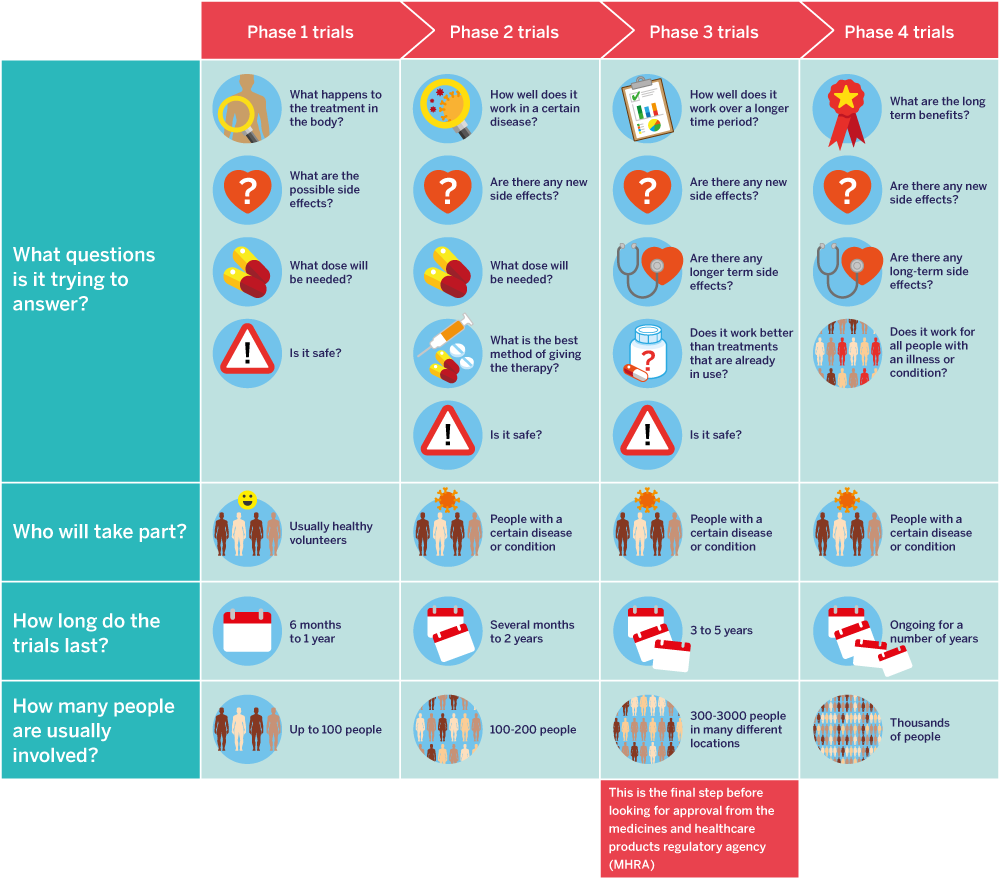
Clinical Trials
A clinical trial compares the effects of one treatment with another. It may involve patients, healthy people, or both.
So, they are not losing out on treatment.

of the people who take part.

for use in their research trial, they need to ask you to give your consent. You should be given written information about what will happen to your tissue sample. Always read the consent form very closely before you sign it.



Some trials look for people who are newly diagnosed or in the early stages of prostate cancer. Others want people with specific symptoms.
Scans.

